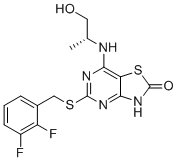
Chemical Structure : AZD-8309
Catalog No.: PC-61471Not For Human Use, Lab Use Only.
AZD-8309 (AZD8309) is a potent and orally bioavailable CXCR2 antagonist that has been proposed to regulate the transmigration of neutrophils.
Bulk size, bulk discount!
E-mail: sales@probechem.com
Tech Support: tech@probechem.com
AZD-8309 (AZD8309) is a potent and orally bioavailable CXCR2 antagonist that has been proposed to regulate the transmigration of neutrophils; significantly reduces MPO in the pancreas and lungs, and reduces intrapancreatic trypsin and elastase activity in caerulein-pancreatitis; also reduces cathepsin B activity and MPO in taurocholate-pancreatitis; also antagonises the CCR2b receptor but it is 10-fold less potent than at CXCR2.
COPD
Phase 1 Clinical
| M.Wt | 384.42 | |
| Formula | C15H14F2N4O2S2 | |
| Appearance | Solid | |
| Storage |
|
|
| Solubility |
10 mM in DMSO |
|
1. Virtala R, et al. Clin Exp Allergy. 2012 Apr;42(4):590-6.
2. Leaker BR, et al. Respir Res. 2013 Dec 16;14:137.
3. Malla SR, et al. Pancreatology. 2016 Sep-Oct;16(5):761-9.










Copyright © 2022 probechem.com. All Rights Reserved. probechem Copyright