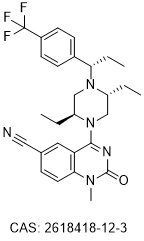
Chemical Structure : BMS-986408
CAS No.:
2618418-12-3
BMS-986408
(BMS 986408, BMS-408)
Catalog No.: PC-24873Not For Human Use, Lab Use Only.
BMS-986408 (BMS-408) is a potent, selective, DAG substrate competitive dual DGKα and DGKζ lipid kinase inhibitor with IC50 of 3 nM and 20 nM respectively, >100-fold selectivity for DGKα over DGKβ and DGKγ.
Bulk size, bulk discount!
Welcome credit card payment!
E-mail: sales@probechem.com
Tech Support: tech@probechem.com
Purity & Documentation Purity:
>98% (HPLC)
Biological Activity
BMS-986408 (BMS-408) is a potent, selective, DAG substrate competitive dual DGKα and DGKζ lipid kinase inhibitor with IC50 of 3 nM and 20 nM respectively, >100-fold selectivity for DGKα over DGKβ and DGKγ.
BMS-986408 inhibits DGKζ and DGKι with similar potency, shows no significant activity for Type II (DGKδ, DGKη, DGKκ), Type III (DGKε, or Type V (DGKθ) family members. BMS-986408 induces the translocation of both YFP-DGKα (EC50=0.026 µM) and YFP-DGKζ (EC50=0.008 µM) from cytoplasm to plasma membrane.
BMS-986408 also induces the degradation of both DGKα and DGKζ, which is ubiquitin and proteosome dependent.
BMS-986408 shows potent amplification of phospho-ERK in both CD4+ (EC50=0.008 µM) and CD8+ (EC50=0.012 µM) T cells and IL-2 production (Donor 1 EC50 0.019 µM; Donor 2 EC50 0.034 µM).
BMS-986408 amplified the antigen-specific activation of naïve mouse OT-1 CD8+ T cells by autologous BMDCs cross-presenting OVA, as measured by proliferation (EC50=0.015 µM) and IL-2 production (EC50=0.022 µM).
BMS-986408 also amplified the antigen specific killing of NY-ESO-1 antigen presenting SAOS-2 cells by human T cells transduced with NY-ESO-1 TCR (EC50=0.001 µM).
BMS-986408 binds to the accessory subdomain of the DGKα and DGKζ catalytic domain.
BMS-986408 broadly amplify T cell–mediated tumor immunity and invigorate both PD-1 immune checkpoint and CAR T-cell therapy.
Physicochemical Properties
|
M.Wt
|
511.59
|
|
Formula
|
C28H32F3N5O
|
|
Appearance
|
Solid
|
|
CAS No.
|
|
|
Storage
|
- Solide Powder
-
-20°C 12 Months; 4°C 6 Months
|
- In Solvent
-
-80°C 6 Months; -20°C 6 Months
|
|
Shipping
|
|
|
Solubility
|
10 mM in DMSO
|
|
Chemical Name/SMILES
|
4-((2S,5R)-2,5-diethyl-4-((S)-1-(4-(trifluoromethyl)phenyl)propyl)piperazin-1-yl)-1-methyl-2-oxo-1,2-dihydroquinazoline-6-carbonitrile
|
References
1. Wichroski M, et al. Cancer Immunol Res. 2025 Jun 12. doi: 10.1158/2326-6066.CIR-25-0156.
Contact Us
sales@probechem.com